First CRISPR gene-editing treatment approved in U.S.
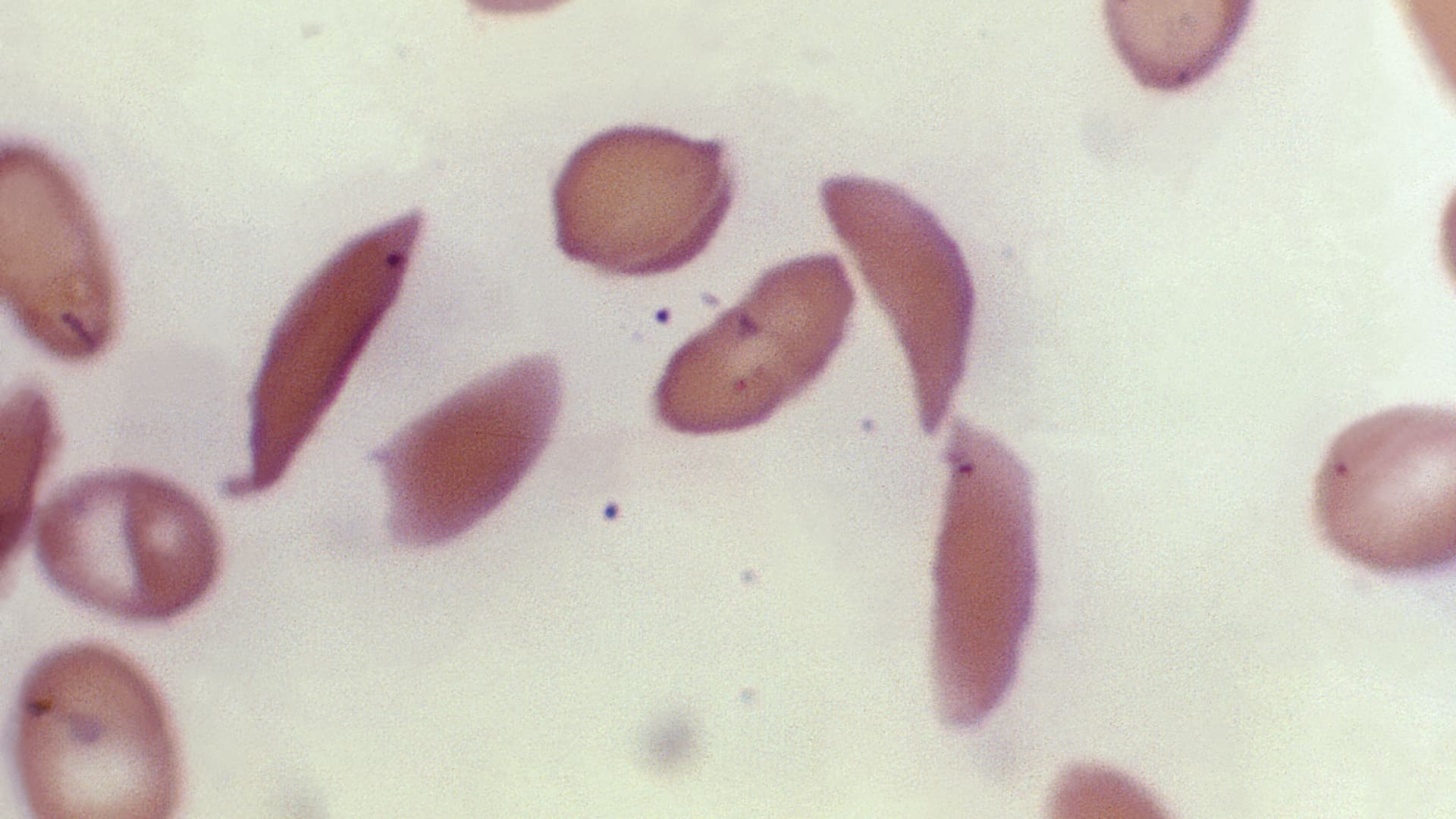
The U.S. Food and Drug Administration on Friday approved the country’s first gene-editing treatment, Casgevy, for use in patients with sickle cell disease. The approval comes about a decade after the discovery of CRISPR technology for editing human DNA, representing a significant scientific advancement. Yet reaching the tens of thousands of people who could benefit … Read more